T.N. Taylor, H. Hass, W. Remy and H. Kerp
Nature, 378: 244
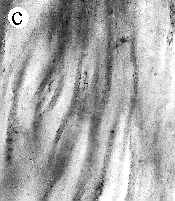
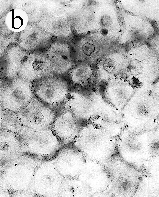
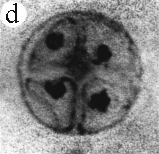
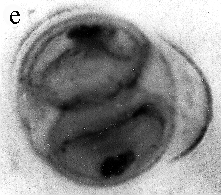
| SIR - We describe here the oldest unequivocal fossil lichen, from thin sections of Early Devonian (400 million years old) Rhynie chert. Lichens are symbiotic associations between a fungus (mycobiont) and a green alga or cyanobacterium (photobiont) which range from mutualism to controlled parasitism¹. The fungus gains a carbon source while the photobiont obtains protection, nutrients and water. Today, lichens can be found in a wide range of habitats, extending from close to the Poles, to deserts and rainforests. The fossil thallus consists of thin bands approximately 1-2 mm thick, in which fungal hyphae are tightly aggregated (figure a). On the surface are numerous pockets containing hyphae in a three-dimensional net (figure b). Nets vary from circular to hexagonal, and average about 25 µm in diameter (figure c). Hyphae are aseptate, possess an irregular surface and are 1-4 µm in diameter. In the centre of each net is a solitary cell (15 µm diameter), or cluster of daughter cells (figure d), representing the photobiont. Each cell is surrounded by a thick envelope of mucilage. Some cells are surrounded by concentric sheaths, the remnants of the envelope (figure e), and these sheaths may contain up to 32 daughter cells. Many photobiont cells contain amorphous contents (figure d-f) which represent stages in protoplast shrinkage and degradation. There is a consistent spatial relationship between cells of the photobiont and those of the mycobiont. At the base of each pocket the photobiont cells are small and solitary; cell size and the number of daughter cells increase towards the upper surface of the thallus. | 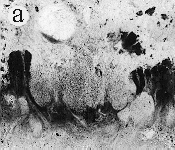
 |
 |
Hyphae are closely aggregated along the edge of the pocket and numerous filaments surround each photobiont cell. Towards the centre of the pocket, hyphae are less aggregated, and in the central region they are often dissociated from the cyanobacterial cells. Some cells of the photobiont possess slight invaginations in the wall that may represent wall appositions or intragelatinous protrusions (figure f). These structures, and the fact that hyphae surround each of the photobiont cells, confirm the physiological interrelationship that existed between the two bionts. The fossil cyanobiont shares numerous morphological features with several extant cyanobacteria (for example, Gloeocapsa, Chroococcidiopsis), in which cells are enclosed in mucilage and produced in plates and spheres². All the daughter cell division patterns in the fossil can be found in extant gloeocapsid colonies. The mycobiont is more difficult to relate to modern fungi because no reproductive structures have been found. Complex microbial communities were present around 3,500 million years ago³, but it is not known whether any consisted of physiological symbioses.To date, the oldest documented fungal symbioses are also found in the Rhynie chert in the form of endomycorrhizae4. The physiological interaction between the Devonian cyanobacterium and fungus resulted in the ability to exploit new ecological niches by competing for space above ground, facilitating the exchange of gas and securing adequate light for the photobiont5. As a result, the Devonian cyanolichens were able to colonize and weather rock, thus contributing to the formation of soil, a necessary step in accommodating increasingly complex and larger terrestrial plants. |
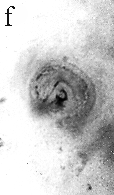 | |||
| a, Longitudinal section of lichen thallus. Black areas are aggregated hyphae; the pocket contains nets and cyanobacterial cells (x 30). b, Section of net showing hyphae surrounding photobiont cells (x 500). c, Detail of fungal hypha (x 800) d, Four of eight daughter cells within a common sheath. Note the degraded protoplast in each cell (x 3000). e, Two daughter cells within a common sheath; outer sheath remnants are only partially preserved (x 3000). f, Cyanobacterial cell showing possible site where photobiont and mycobiont interact physiologically (x 2000). | |||
 These photos can also be viewed
enlarged. These photos can also be viewed
enlarged.
| |||
1. Ahmadjian, V. The Lichen Symbiosis (Wiley, New York, 1993).
2. Büdel, B. & Rhiel, E. Archs Microbiol. 143, 117-121 (1985).
3. Awramik, S. M. Photosynth. Res. 33, 75-89 (1992).
4. Taylor, T. N., Remy, W., Hass, H. & Kerp, H. Mycologia 87,
560-573 (1995).
5. Honegger, R. New Phytol. 125, 659-677 (1993).